Analysis: Lessons from Novartis’ Approach to Population Health Management & Innovative Financing Models
Novartis, leader in the Population Health Management approach to expanding patient access to medicines, initiated a program in collaboration with NHS England to reduce low-density lipoprotein levels in patients with atherosclerotic cardiovascular disease, targeting up to 300,000 patients over three years. While the approach has seen success, challenges persist in its implementation, including technical, mindset, and regulatory barriers.
What is Population Health Management (PHM) and why is it important?
PHM is an innovative approach to expanding patient access to medicines based on models that develop proactive, rather than reactive, care. It is data-driven, collaborative, and targeted at those conditions with the greatest unmet needs¹. The benefits of PHM include preventing community ill-health, addressing health inequalities, and improving population outcomes. Key to PHM are financial contracts that enable latent and/or large populations to be diagnosed and treated at an affordable net price.
PHM is particularly beneficial for chronic conditions, where an ability to be diagnosed and treated in primary care, using high budget impact and/or high-cost drugs, is available. A good example could be the use of PHM for new obesity medicines like Novo Nordisk’s Wegovy. Omar Ali, Head of Payers at Verpora said, “in obesity, the payer’s traditional mindset is that most patients return to their original weight within a couple of years post-treatment. Payers therefore ask, is it affordable to pay for the many failures to find a few winners? To gain widescale adoption of new obesity medicines, this psychological barrier will need to be overcome and PHM could play a significant role”.
Who led the way and how?
The Swiss pharmaceutical group, Novartis, have established themselves as leaders in PHM. Their innovative partnerships and collaborations provide many lessons.
One of the most notable examples with NHS England in 2021, aimed at reducing low-density lipoprotein (LDL) levels in patients with atherosclerotic cardiovascular disease (ASCVD). The approach supported the identification, treatment, and monitoring of high-risk patients with ASCVD. As a result, it was expected that up to 300,000 people over three years² could be treated with Leqvio (inclisiran) at an affordable net price. To aid patient identification, every Electronic Medical Record system integrated a digital tool, enabling the general practice patient population to be re-stratified for review.
This approach to population health management was highlighted in a 2022 Novartis investor presentation³. The company said they needed to "connect the dots through the system to make this a 'systems approach' and not just a classical launch”. The presentation also stated that by partnering with NHS England, Novartis could reach a level of access they wouldn't usually get.
By September 2022, just under 95% of prescribing formularies in the UK had broad access to Leqvio, and approximately 14% of GP practices in the UK had ordered and used the product. The limited uptake in GP practices was due to several implementation challenges including:
A need for the restructuring of local lipid management pathways.
Primary care physicians requiring training on the administration of injectables.
The COVID-19 pandemic creating a substantial patient backlog.
Given these factors, it’s easy to see why a national strategy doesn’t always immediately translate to successful implementation on the ground.
We’ve already seen that PHM measures carried out locally can be such an impactful tool in actively preventing community ill-health and addressing health inequalities. We have seen improved efficiencies through PHM measures, with patient outcomes improved more holistically and not just in terms of a specific medical diagnosis. Now is the time for us to combine efforts to push for widespread adoption of this patient-centric approach.¹
Marie-Andrée Gamache, Country President and Managing Director, UK and Ireland, Novartis
The group’s collaboration with NHS England is part of a more significant movement. In 2022, the company sponsored a paper with the London School of Economics on ‘The Promise of Population Health Management in England: From Theory to Implementation’⁴. The article outlined the challenges and enablers of PHM and provided policy recommendations for healthcare systems looking to implement these approaches.
One of the challenges outlined in the paper was the siloed nature of the NHS, which makes progress difficult to track. The other implementation barriers highlighted were:
Technical gaps: Data interoperability, inequality measurements, incentives, collaboration, and limited digital and analytical skills available within the NHS.
Mindset gaps: Proactive vs reactive attitudes to care and political mindset.
Regulatory gaps: The speed of innovation and unintended consequences of technologies.
In response to this, the paper set out a series of enablers for PHM, including:
Communication between the system and the public.
The ethos of those working within the NHS.
Digital transformation.
The unitary system of the NHS allowing shared information and learning between systems.
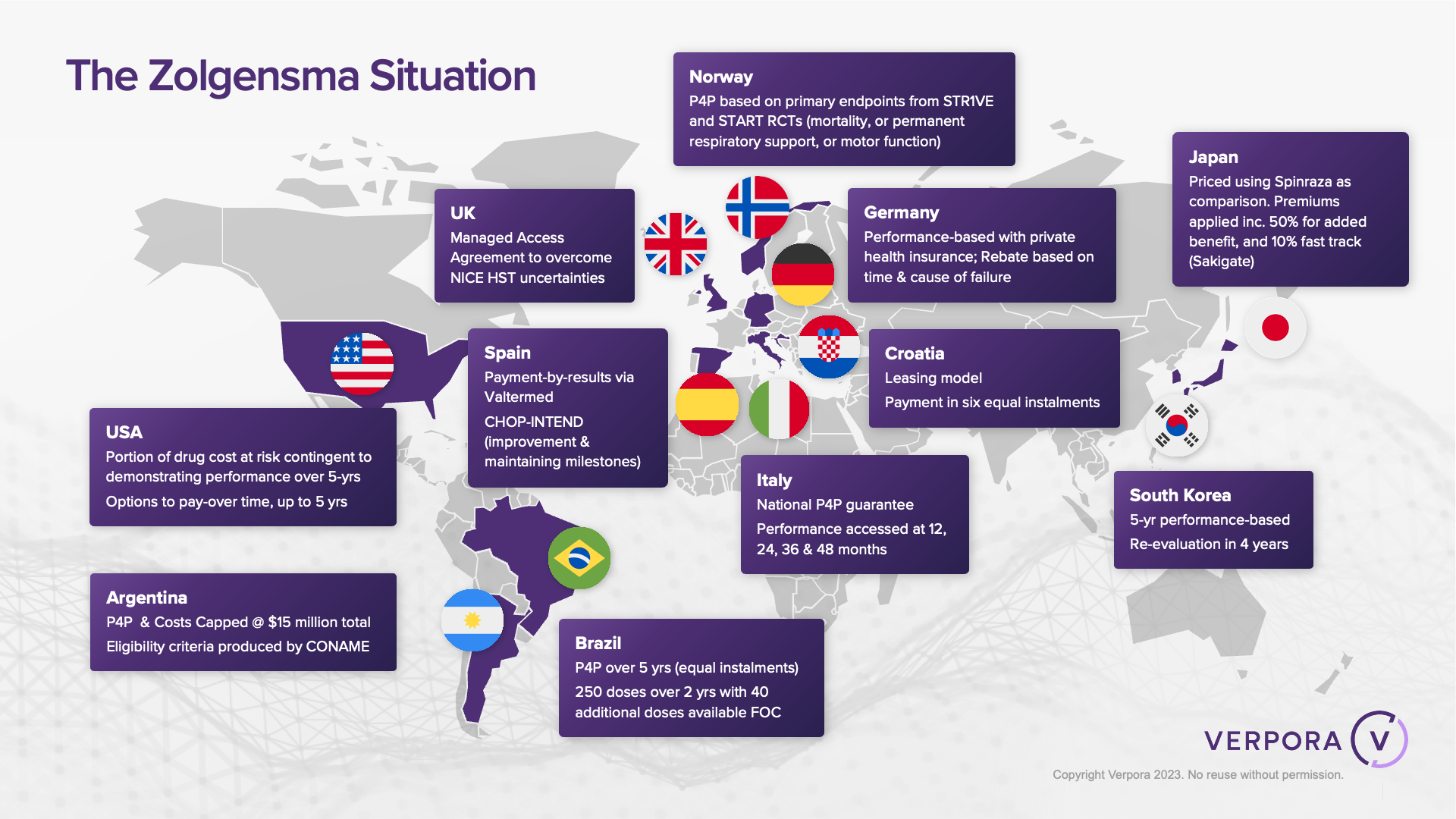
Novartis also leads the way in establishing innovative contracting models linked to population health management. An example is Zolgensma in Brazil, where in October 2022, an outcomes-based, pay-over-time agreement was signed with the National Health Service (SUS)⁵. The SUS will receive access to up to 250 doses of Zolgensma in the first two years, with the possibility of an additional 40 doses at no extra cost. The cost of treatment is covered through a five-year, outcomes-based agreement in the form of annuity instalments, with payment tied to patient outcomes and clinical milestones. Population health was considered within the agreement, where an additional discount of 3% on every dose would be given if the financial savings were redirected to the screening of spinal muscular atrophy (SMA) in children.
Where is Population Health Management Today?
At a global-level, Zolgensma has 13 published innovative financial agreements across 11 countries⁵. This shows a concerted approach to launching with innovative contracts and has resulted in a strong adoption of the products across 40 markets.
In the UK, the impact of the atherosclerotic cardiovascular disease PHM agreement with the English NHS is interesting. Use of inclisiran (administered twice yearly) in primary care has increased from 218 items in July 2022 to 1,106 in July 2023⁶. This would suggest that the original target of 300,000 patients over three years may be ambitious. Ambitions to shift diagnosis and treatment from secondary to primary care seem to have been hindered, with external reports suggesting that challenges have included⁷:
General Practice physicians unwilling to prescribe, seeing treatment as a secondary care issue
In December 2021 the Royal College of GPs & the British Medical Association formally discouraged family doctors from initiating or overseeing treatment
However, the approach would seem an overall success for the manufacturer. The Financial Times reported the Swiss group as stating they had already been a “significantly faster patient uptake of inclisiran in the UK relative to the launch of other lipid-management therapies used in combination with statins.”⁷
An Innovative Mindset to PHM
As new drugs for chronic conditions are launched, the ability for payers to treat meaningful populations and pay based on value, has a clear place in the pricing landscaping. Lessons can be learnt and in addition to the recommendations provided in the LSE report⁴, Verpora believes the core challenges manufacturers face include:
Shifting traditional internal mindsets to adopt new ways of operating
Ensuring all external stakeholders are identified, consulted, and are bought into the proposed strategy before it is implemented.
PHM is an iterative process for which there is no single globally accepted 'rulebook', it’s up to forward thinking manufacturers to go write it!
Contributors
Nick Merryfield
CEO & Head of Business Consulting, Verpora
LinkedIn
After sales and leadership roles at SmithKline Beecham and Boehringer-Ingelheim, Nick started his career in consulting by founding Infonetica Ltd. and Pharmaceutical Field Ltd. Respectively, each organisation helped the UK pharmaceutical industry improve clinical research activity and manage the evolving payer landscape of the late 1990s. After the sale of the businesses, Nick formed and led NHiS Ltd. During this time, he was responsible for spearheading ground-breaking work on using Hospital Episode Statistics and the 2001 Freedom of Information Act to understand the relationship between drug intervention and outcomes.
Following the acquisition of NHiS in 2013, Nick helped found Verpora Ltd, a global specialist consultancy in the field of value-based contracting. Joined by leading pharmacists and data analysts, the organisation now focuses on contract creation and the improvement of drug usage data by indication to underpin value-based negotiations.
Nick is ABPI qualified and a former Board Director of the British Healthcare Business Intelligence Association (BHBIA).
Adam Buckler
MPharm, PGDip GPP, Senior Business Consultant Verpora
& Former Oncology Pharmacist, Royal Marsden Hospital, London
LinkedIn
Adam is an experienced pharmacist having worked in the UK NHS for over ten years in acute Trusts and an internationally renowned oncology/ haematology centre. Acting as the lead pharmacist for Electronic Prescribing & Medicines Administration (EPMA) and being responsible for the Education and Training of pharmacists, Adam developed a breadth of experience. With an expertise in healthcare data systems and capabilities, Adam is an expert in the field of electronic prescribing. At Verpora Adam leads Innovative Contracting consultancy projects and client contracting capability program builds. Adam is a certified member of the BHBIA & registered with the GPhC.
Omar Ali
BSc(Hons)Pharm DipClinPharm MRPharmS ACPP
Head of Payers, Verpora
LinkedIn
Qualified with a hospital pharmacy background Omar started as a formulary payer within the managed care setting. For over 15 years he sat on payer P&T committees with responsibility for the allocation of drug budget, access, and reimbursement. At a national-level, Omar served as a NICE national HTA adviser for nine years across three committees evaluating cost-effectiveness, budget impact/ affordability and value-based agreements. Omar has spent the last ten years leading a global network of over a hundred current & former payers that specialise in value-based contracting. Omar’s team provides the inside track on creating innovative contracts that will work. Omar has embarked on a Ph.D. entitled, “Value-Based Pricing & Outcomes-Based, Innovative Contracting of New Medicines”. He is a visiting lecturer in value-based contracting at the University of Portsmouth, UK.
Sources
https://www.novartis.com/uk-en/about/partnerships/population-health-management - accessed 22/03/2023
https://www.novartis.com/news/media-releases/world-first-agreement-between-novartis-and-nhs-enables-broad-and-rapid-access-first-class-cholesterol-lowering-medicine-leqvio-vinclisiran - accessed 22/03/2023
https://www.novartis.com/investors/event-calendar/meet-novartis-management - accessed 22/03/2023
Verpora Innovative Contracting Tracker of 800 published innovative and value-based contracts - https://www.verpora.com/research-and-intelligence
https://openprescribing.net/chemical/0212000AM/ - accessed 6/9/23
Novartis scraps drug trial in blow to UK life sciences ambitions, Financial Times, https://www.ft.com/content/d7685eaa-5635-4e64-a390-b48908874bfe - accessed 22/3/23
Disclaimer
Content in this article is based on secondary market research using externally sourced data available in the public domain. Opinions and commentary are those of the authors and do not reflect views of any commercial organisation or government body mentioned in the article. For any questions relating to the article please contact adam.meads@verpora.com.